Coppinger Reveals More Info on Whyte's Dirty Test!
Collapse
-
-
Comment
-
Just read Hauser's article again.
No where does he say the test that popped dirty took place on the 17th…. Just that the results came back on the 17th. You don't test and get the results back on the same day. Just not possible.The positive test result is believed to have come back on July 17.Comment
-
You don't know. You see one metabolite mentioned as being 19 days and you assuming all other must be short. Even though that source is from 2006.No he wasn't. And even if that were true he was also caught with short detection window metabolites. lol, you are repeating things you don't effin know. For ex. even I was unsure till I made sure.
https://www.boxingscene.com/forums/s...01&postcount=9

Stop acting like you're some expert when you aren't.Comment
-
Comment
-
Comment
-
For me it matters for VADA to get in too, that means the WBC would have to be notified because they obviously sanctioned the fight, that meant Team Rivas also would have to be notified. Which meant the fight wouldn’t have gone ahead and Whyte would have been banned right away.
Now we have a scenario where a dirty fight (Allegedly) has been allowed to fight without notifying his opponent. Looking at it from the outside it looked like a cover up amongst the parties involved.Comment
-
I'm gonna put you on ignore after this. Enjoy your ownage.
https://www.ncbi.nlm.nih.gov/pubmed/2031859
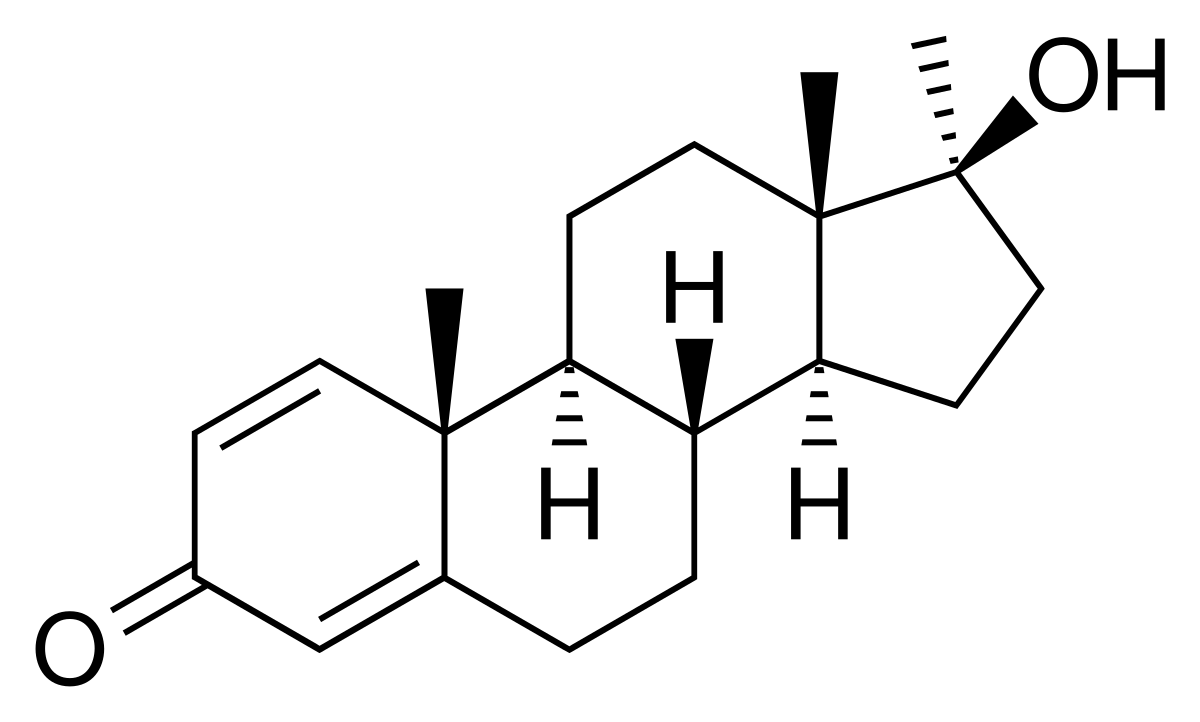
After oral administration of metandienone (17 alpha-methyl-androsta-1,4-dien-17 beta-ol-3-one) to male volunteers conjugated metabolites are isolated from urine via XAD-2-adsorption, enzymatic hydrolysis and preparative high-performance liquid chromatography (HPLC). Four conjugated metabolites are identified by gas chromatography-mass spectrometry (GC/MS) with electron impact (EI)-ionization after derivatization with N-methyl-N-trimethyl-silyl-trifluoroacetamide/trimethylsilyl-imidazole (MSTFA/TMS-Imi) and comparison with synthesized reference compounds: 17 alpha-methyl-5 beta-androst-1-en-17 beta-ol-3-one (II), 17 alpha-methyl-5 beta-androst-1-ene-3 alpha,17 beta-diol (III), 17 beta-methyl-5 beta-androst-1-ene-3 alpha,17 alpha-diol (IV) and 17 alpha-methyl-5 beta-androstane-3 alpha,17 beta-diol (V). After administration of 40 mg of metandienone four bis-hydroxy-metabolites--6 beta,12-dihydroxy-metandienone (IX), 6 beta,16 beta-dihydroxy-metandienone (X), 6 beta,16 alpha-dihydroxy-metandienone (XI) and 6 beta,16 beta-dihydroxy-17-epimetandienone (XII)--were detected in the unconjugated fraction. The metabolites III, IV and V are excreted in a comparable amount to the unconjugated excreted metabolites 17-epimetandienone (VI), 6 beta-hydroxy-metandienone (VII) and 6 beta-hydroxy-17-epimetandienone (VIII). Whereas the unconjugated excreted metabolites show maximum excretion rates between 4 and 12 h after administration the conjugated metabolites III, IV and V are excreted with maximum rates between 12 and 34 h.
CIBA filed for a U.S. patent in 1957,[22] and began marketing the drug as Dianabol in 1958 in the U.S.
Detection in body fluids
Metandienone is subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites are detectable for up to 3 days

Comment
-
I found theres a lot of lawyers on here to all of a sudden and /or claiming to hold the highest degrees , who knew ? lolComment
-
I'm gonna put you on ignore after this. Enjoy your ownage.
https://www.ncbi.nlm.nih.gov/pubmed/2031859
After oral administration of metandienone (17 alpha-methyl-androsta-1,4-dien-17 beta-ol-3-one) to male volunteers conjugated metabolites are isolated from urine via XAD-2-adsorption, enzymatic hydrolysis and preparative high-performance liquid chromatography (HPLC). Four conjugated metabolites are identified by gas chromatography-mass spectrometry (GC/MS) with electron impact (EI)-ionization after derivatization with N-methyl-N-trimethyl-silyl-trifluoroacetamide/trimethylsilyl-imidazole (MSTFA/TMS-Imi) and comparison with synthesized reference compounds: 17 alpha-methyl-5 beta-androst-1-en-17 beta-ol-3-one (II), 17 alpha-methyl-5 beta-androst-1-ene-3 alpha,17 beta-diol (III), 17 beta-methyl-5 beta-androst-1-ene-3 alpha,17 alpha-diol (IV) and 17 alpha-methyl-5 beta-androstane-3 alpha,17 beta-diol (V). After administration of 40 mg of metandienone four bis-hydroxy-metabolites--6 beta,12-dihydroxy-metandienone (IX), 6 beta,16 beta-dihydroxy-metandienone (X), 6 beta,16 alpha-dihydroxy-metandienone (XI) and 6 beta,16 beta-dihydroxy-17-epimetandienone (XII)--were detected in the unconjugated fraction. The metabolites III, IV and V are excreted in a comparable amount to the unconjugated excreted metabolites 17-epimetandienone (VI), 6 beta-hydroxy-metandienone (VII) and 6 beta-hydroxy-17-epimetandienone (VIII). Whereas the unconjugated excreted metabolites show maximum excretion rates between 4 and 12 h after administration the conjugated metabolites III, IV and V are excreted with maximum rates between 12 and 34 h.
CIBA filed for a U.S. patent in 1957,[22] and began marketing the drug as Dianabol in 1958 in the U.S.
Detection in body fluids
Metandienone is subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites are detectable for up to 3 days

I asked for a link saying the specific metabolites that where found in Whyte's test are only detected within 3 days. You post everything but that.
Wannabe expert.....
Comment


Comment